 Dirofilaria spp are parasitic spirurid nematodes which causes vascular disease in cats throughout temperate and tropical countries worldwide. D. immitis is the primary worm of this genus which causes heartworm disease in cats[2].
Dirofilaria spp are parasitic spirurid nematodes which causes vascular disease in cats throughout temperate and tropical countries worldwide. D. immitis is the primary worm of this genus which causes heartworm disease in cats[2].
Infection is a relatively rare disease of cats (rates of infection at 5-20 of that of dogs[3]), but is increasingly diagnosed parasite in feline practise due to heightened awareness of the disease and improved diagnostic methods. Many cats are subclinically infected and infection tends to be self-limiting.
Clinically affected cats may present at veterinary clinics with a wide range of clinical signs, such as chronic coughing, laboured breathing and vomiting, however, many infected cats die suddenly without any premonitory signs. Feline heartworm disease is clinically challenging on a number of different levels. Diagnostic confirmation usually requires a combination of tests and treatment is most often limited to symptomatic therapy as curative medical and surgical treatments place the feline patient at significant risk. Safe and effective prophylactic drugs which kill a number of life-cycle stages are readily available.
Pathophysiology
Feline infection can occur at any age and immunosuppression is not a prerequisite for infection. Indoor and outdoor cats are equally represented. Male cats were thought to have a higher prevalence of infection than female cats but a relatively large retrospective study disputes the point. Acute lung injury is a major contributing factor to the initiation of clinical signs. It is hypothesised that the arrival of fifth-stage larvae in the lungs and the death of the adult are the most likely stages of the life-cycle to be associated with clinical signs. After an initial host response, the signs may abate and become subclinical. In chronic cases, perivascular reaction and evidence of thrombus formation with recanalisation are noted.
D. immitis has evolved an array of specific molecular strategies to evade host immune attack. Marked difference in the surface properties of the third and fourth larval stages may delay potentially destructive immune responses to these stages in the host. Clinical and laboratory investigations have suggested that the acellular nature of the D. immitis cuticle and its thrombo-resistant surface properties allow the parasite to circumvent the host’s immune response and improve long-term survival. Another part of D. immitis capacity to evade host immune responses is due in part to the presence of Wolbachia spp, a bacteria that lives symbiotically within the reproductive tract of adult D. immitis heartworms. This bacteria co-evolved with most filariids including those which infect humans, such as Wuchereria bancrofti and Brugia malayi (Elephantiasis), and Onchocerca volvulus (River blindness). Elimination of the bacteria Wolbachia from filarial nematodes generally results in either death or sterility of the heartworm adults. Consequently, current strategies for control of filarial nematode diseases include elimination of Wolbachia via the simple doxycycline antibiotic rather than far more toxic anti-nematode medications.
There is evidence that Wolbachia, an endosymbiontic bacterium present in D. immitis, may play a role in immunopathogenesis of heartworm disease. Heartworm-infected cats can be exposed to Wolbachia when larvae, or adult worms, are killed; when Wolbachia are released with microfilariae from the uterus of females; and possibly through the excretory system of both male and female worms. One recent study demonstrated a strong IgG response against the surface protein of Wolbachia in heartworm-infected cats, leading to the suggestion that bacteria could play an important role also in the inflammatory reaction which is characterises the heartworm infection in cats.
Clinical Signs
Clinical presentations of feline heartworm disease vary widely in severity and include acute death, chronic coughing or intermittent dyspnoea, and asymptomatic infections. In one stody of 50 cases, asymptomatic infections were diagnosed incidentally in 28% of cats. Cats infected with immature worms, or as few as one adult worm may show clinical signs. At presentation, clinical signs are most commonly related to respiratory tract, with dyspnoea and coughing most often observed. Vomiting is a relatively common finding, reported in about one third of cases. Neurological signs, including syncope, collapse, blindness and vestibular signs, may also occur, probably in association with aberrant larval migration through the brain. The initial host response of diffuse pulmonary infiltrate and resultant clinical signs occurs most frequently about 4-7 months after infection and is usually followed by a subclinical stage. However, the subsequent death of adult heartworms may cause additional severe signs, such as acute collapse and death. [[Congestive heart failure is a rare clinical signs. Acute death syndrome due to D. immitis is commonly reported.
Diagnosis
A thorough diagnostic approach using a combination of tests is necessary in the diagnosis of feline heartworm disease because of the low worm burdens and light antigen load. Serology available for the diagnosis of feline heartworm disease includes serum antigen and serum antibody tests. In the serum antigen test, an enzyme-linked immunosorbent assay (ELISA) detects a protein found primarily in the reproductive tract of the female worm. This test may lack sensitivity due to low worm numbers and the possibility of infection solely with male worms and so it is not recommended as a screening test for feline heartworm infections[4]. However, the rate of false-positive results with antigen serology is low, so that a positive result generally indicates a current infection. ELISA antibody tests are available commercially as screening tests for cats when there is an index of suspicion for heartworm infection. The specificity of antibody tests may be compromised because they detect exposure to migrating heartworm larvae and will also be positive in cats with previous heartworm infections. False-negative antibody test results were previously considered rare, but in two independently conducted studies, 14% of infected cats have negative antibody test results. Another more recent study reported that more than 20% of antigen-positive heartworm-infected cats were antibody negative. Combining the results of serum antigen and antibody tests achieces higher sensitivity and specificity than by using either test alone. Sensitivities of up to 100% and specificities of up to 99.4% were reported in one study, when antigen and antibody tests were used in combination[5].
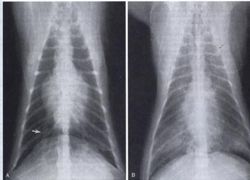
Thoracic radiography is a valuable tool for diagnosis and case monitoring in feline heartworm disease. Radiographic changes associated with feline heartworm disease include enlargement, blunting and tortuosity of the peripheral pulmonary arteries, especially on the right side in the dorsoventral (DV)/ ventrodorsal (VD) views; cardiomegaly and right ventricular enlargement; and patchy focal or diffuse pulmonary parenchymal changes. A mean ratio of greater than 1.6 for the width of the right pulmonary artery (at the causal border with rib 9) to the width of rib 9 in the DV or VD view has been reported in association with feline heartworm disease[6].
Objective measurement of radiographic heart size in heartworm-infected cats showed that mean heart size on lateral radiographs was significantly larger than the reference value for vertebral heart score. There was also a significant positive correlation between mean diameter of the caudal vena cava and heart size on lateral radiographs of infected cats. Alterations to structures visible on thoracic radiographs may occur less consistently in feline heartworm disease than in canine heartworm disease and the absence of radiographic abnormalities does not exclude a diagnosis of heartworm disease in cats.
Echocardiography is a useful adjunctive test in cats in which there is a suspicion of heartworm disease, but antigen test results are negative. One retrospective study of heartworm-infected cats reported that heartworm were detectable by the use of echocardiography in 17 of 43 cats, most often in the pulmonary arteries, but also in the right ventricle, right atrium and caudal vena cava. Heartworm infection was diagnosed exclusively by the use of echocardiography in five cats in which antigen test result was negative. The sensitivity of echocardiography for the detection of heartworm infections in cats is highly operator-dependent and some experienced investigators have reported up to 100% sensitivity. It is possible to obtain false-positive results when assessing cats at risk for heartworm using echocardiography, due to occasional presence of linear densities that mimic filariae. These densities are found when the main pulmonary artery branches and their cause is unknown, but they are presumed to be sonic reflections from the pulmonary artery wall.
Necropsy confirmation of heartworm infection has been used as the standard for determining heartworm status in dogs, but routine necropsies may miss ectopic infections, which are more common in cats. Precardiac infections may also cause clinical signs in cats, but are difficult to confirm on necropsy. However, necropsy is still the method against which the performance of other tests is judged.
Treatments
Medicines
- Doxycycline orally once daily (eliminating Wolbachia spp and hence sterilising adult D. immitis).
- Adulticidal treatment of cats with heartworm infection is associated with significant risk. In addition to toxicity and reported lack of efficacy of heartworm adulticidal agents, adulticide treatment of heartworm-infected cats results in nearly universal and often fatal pulmonary thromboembolism with necrosis. Thiacetarsemide is believed to be a less effective adulticide in cats than in dogs (reported efficacy <70%) and cats are more likely to manifest adverse reactions to this arsenical agent. The safety and efficacy of melarsomine in heartworm-infected cats are being investigated, but preliminary data indicate that its efficacy is only about 36% against adult heartworm in cats. For these reasons and because heartworm infection is often self-limiting, infected cats are frequently managed only with supportive treatment (corticosteroids, bronchodilators, and anti-emetics).
- Prednisolone in diminishing doses is often effective for infected cats with radiographic evidence of lung disease, or infected cats that display clinical signs. An empirical oral regimen is 2 mg/kg/day, declining gradually to 0.5 mg/kg every other day by 2 weeks and then discontinued after an additional 2 weeks. At that time the effects of treatment should be assessed based on the clinical response and/or thoracic radiography. This treatment may be repeated in cats with recurrent clinical signs. However, conservative management is not without risk, as the acute death syndrome may occur without premonitory signs and in the presence of only one filaria[7].
Surgical treatment
Surgical removal of heartworm is feasible and effective in symptomatic cats with echocardiographically visible filariae in the right heart and main pulmonary arteries. Transjugular catheterisation and removal of heartworm using rigid or flexible alligator forceps, horsehair brushes, endoscopy grasping forceps or basket-type retrieval catheters have been well described in the literature. Other more invasive techniques including right auricular entry into the heart and main pulmonary arteriotomy have also been developed. Heartworm extraction often results in rapid and dramatic clinical improvement. However, accidental damage to the heartworm during extraction procedure can result in shock-like signs and death.
Currently, there are no products in the United States approved for the treatment of feline heartworm infection. Most cats with heartworm infection that are not demonstrating clinical signs are allowed the time for a spontaneous cure to occur. If there is evidence of disease in the lungs and their blood vessels consistent with feline heartworm infection, such cases (possibly in the early stage) can be monitored with chest X-rays every six to twelve months, as needed.
Cats with severe manifestations of feline heartworm disease may require additional supportive therapy, and may benefit from intravenous fluids, oxygen therapy, cage confinement, bronchodilators (which expand the air passages of the lungs), cardiovascular drugs, antibiotics and nursing care.
Necropsy findings
Most pathological findings in heartworm-infected cats involve the lungs. Affected cats develop villous endarteritis and muscular hypertrophy of the pulmonary arteries and arterioles. There may be formation of elevated ridges projecting above the surface of arteries/arterioles. Lobar and medium-sized pulmonary arteries are similarly affected, with villus-like structures that protrude into the lumen and partially obliterate affected vessels. Multiple areas of infiltration of eosinophils, lymphocytes, macrophages and plasma cells are observed in the intima and mild multifocal accumulation of macrophages are present in the alveoli of most cats. Cats infected with Toxocara cati and Aelurostrongylus abstrusus may develop similar pulmonary arterial pathology.
In one study, pulmonary arterial hypertrophy were demonstrated for 2 years after infection with A. abstrusus and it was suggested that these changes may persist for the entire life of the cat. Confusingly, pulmonary artery medial hypertrophy and hyperplasia have also been reported in specific pathogen free cats, with one study reporting the same frequency in both specific pathogen-free and conventional cats, indicating that pulmonary parasitic infection is not a prerequisite for the condition. Increased muscular thickness of the medial layer of the pulmonary arteries must also be differentiated from vasoconstriction, as both can appear similar microscopically. This is achieved by calculating the area of the median on cross-section in a number of large and small arteries in a section of lung. The resultant figures are compared with reference ranges for normotensive pulmonary arteries and those with moderate and severe pulmonary hypertension[8].
Prophylaxis
There are currently four macrolytic lactone drugs registered for feline heartworm prophylaxis – moxidectin, ivermectin, milbemycin oxide and selamectin. These products are a safe and convenient option for cats living in areas where canine heartworm disease is endemic and exposure to infective mosquitoes is possible. Additionally, depending on the active ingredient, these products protect cats from a variety of common endo-and ecto-parasites. Indoor-only housing status is not a reliable method of prevention of infection, as the home environment may not provide an effective barrier to the entry of mosquitoes. One retrospective study reported that 27% of infected cats were kept exclusively indoors.
Prolonged administration of macrolytic lactones has a ‘reachback effect’, i.e. they can kill young larvae, older larvae, immature or young adults, and/or older adult filariae. This is advantageous in cases of owner compliance failure, or when heartworm infection status is unknown at the time that prophylactic treatment is commenced. Efficacy of 95% or more requires dosing for 9-30 months, and older worms are difficult to kill. Of the various macrocytic lactone, ivermectin has the most potent combination of clinical prophylaxis, reachback activity and adulticidal activity; milbemycin oxime has the least; and selamectin and moxidectin injectable lie somewhere in between. The unique effects of ivermectin are related to the age of the heartworms at the initiation of treatment. The earlier treatment is started, the more stunted and smaller the worms and the shorter their survival time.
References
- ↑ Atkins, CE & Litster, AL (2006) Heartworm disease. In August, JR (Ed): Consultations in feline internal medicine. Vol 5. Elsevier Saunders, Philadelphia. pp:323-330
- ↑ Bowman, DD et al (2003) Feline clinical parasitology. Iowa University Press, Iowa, pp:331-338
- ↑ Ryan, WG & Newcomb, KM (1996) Prevalence of feline heartworm disease – a global review. In Soll, MD Knight DH (Eds): Proc Am Heartworm Symp ’95, Batavia, IL American Heartworm Society pp:79-86
- ↑ Snyder, PS et al (2000) Performance of serologic tests used to detect heartworm infection in cats. J Am Vet Med Assoc 216:693-700
- ↑ Akins, CE (1998) Veterinary CE advisor: heartowrm disease: an update. Vet Med 93(12):2-18
- ↑ Selcer, BA et al (1996) Radiographic and 2-D echocardiographic findings in eighteen cats experimentally exposed to D. immitis via mosquito bites. Vet Radiol Ultrasound 37:37-44
- ↑ Goodman, DA et al (1996) Evaluation of a single dose of melarsomine dihydrochloride for adulticidal activity against Dirofilaria immitis in cats. Proc Am Assoc Vet Parasitol 41:64
- ↑ Litster, AL & Atwell, RB (2008) Feline heartworm disease: a clinical review. JFMS 10:137-144